 Image 1 of 1
Image 1 of 1


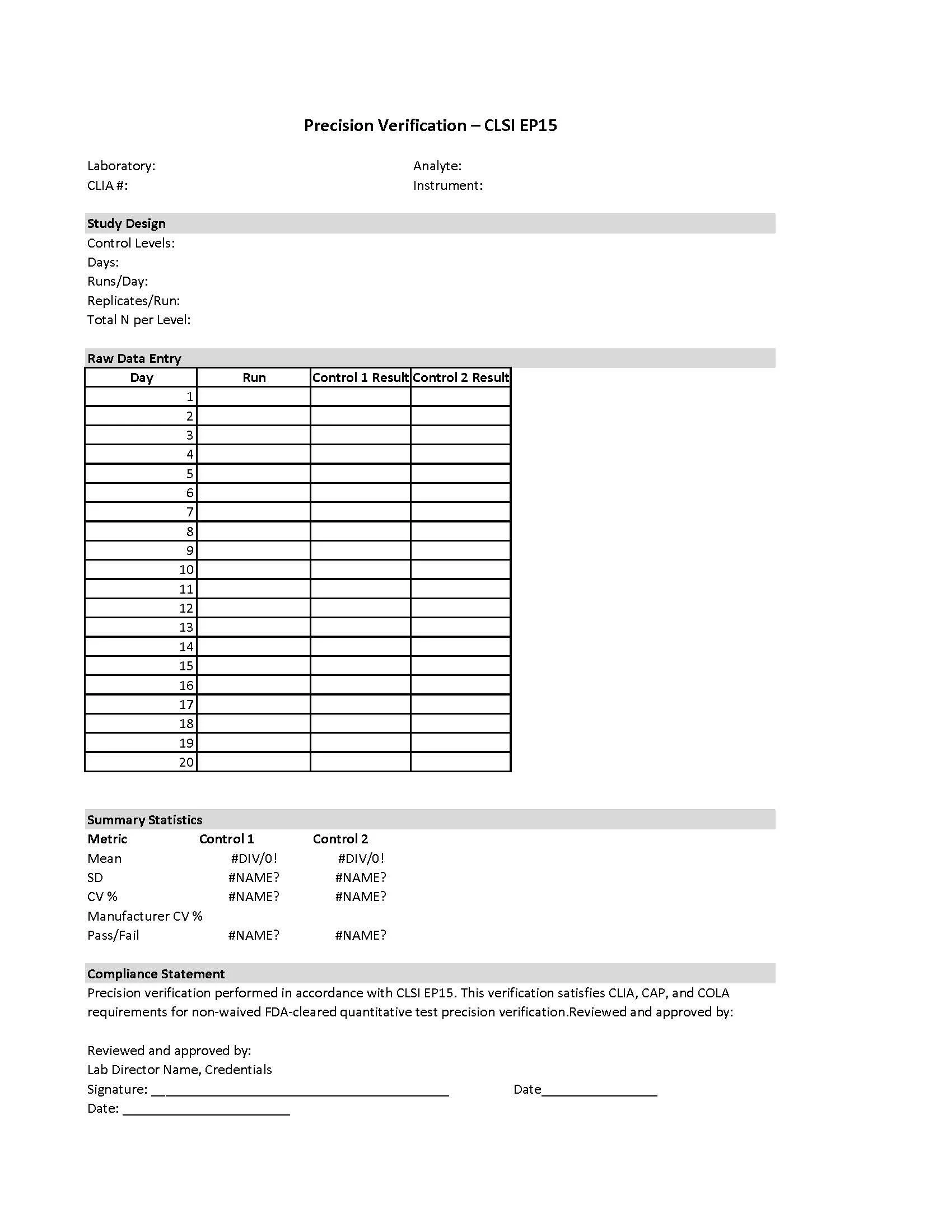
Precision Verification Template – CLSI EP15 (CAP/CLIA/COLA/TJC Aligned)
Designed for small laboratories and physician office laboratories (POLs), this Excel-based template supports user verification of precision for FDA-cleared, non-waived quantitative tests in accordance with CLSI EP15.
Precision verification is a required component of method verification, yet many smaller labs perform these studies infrequently and do not need the cost or complexity of enterprise statistical software. This template provides a practical, cost-effective alternative, offering the structure, calculations, and documentation support needed to perform CLSI-aligned precision verification without ongoing software subscriptions.
The study design incorporates multiple runs and multiple days, capturing both within-run and between-run sources of variability through its structure and allowing laboratories to evaluate overall within-laboratory precision, consistent with CLSI EP15 expectations. Separate variance component analysis is not required for EP15 verification.
The template includes clearly organized data entry fields, automated calculations, and objective acceptance logic to support precision verification during new test implementation, instrument changes, or other verification activities.
Key Features
Structured worksheet supporting CLSI EP15 precision verification
Multi-day, multi-run design that incorporates within-run and between-run variability
Automated calculations for:
Mean
Standard deviation (SD)
Coefficient of variation (CV%)
Automatic PASS / FAIL determination based on manufacturer precision claims
Clearly defined study design section for inspection clarity
Separate Instructions tab explaining study design, data entry, and interpretation
Clean, professional format suitable for CLIA, CAP, TJC, and COLA inspections
Designed by a CLSI-experienced Clinical Laboratory Scientist and Laboratory Director
Reusable for multiple analytes and instruments
Why This Template Makes Sense for Small Labs and POLs
Many small laboratories and physician office labs do not need to maintain annual licenses for statistical software just to perform occasional precision verification studies. This template is designed for laboratories that need reliable, CLSI-aligned documentation without unnecessary overhead.
For labs that:
Perform verification studies periodically rather than continuously
Operate with limited testing volume
Value practical, inspection-ready documentation
this template offers the right level of rigor without unnecessary cost or complexity.
Intended Use
This spreadsheet is intended to assist clinical laboratories with internal documentation and evaluation of precision verification studies for moderate- and high-complexity testing, when verification is appropriate for FDA-cleared test systems and within the laboratory’s scope of practice.
This template supports precision verification (CLSI EP15) and is not intended to replace full precision characterization studies described in CLSI EP05.
Compatibility
Microsoft Excel (desktop version recommended)
Not designed for Google Sheets or Excel Online
License
Single-laboratory use
Redistribution, resale, or sharing outside the licensed laboratory is prohibited
Disclaimer
This template is provided as an educational and documentation support tool and does not replace professional judgment or regulatory requirements. Laboratories are responsible for ensuring compliance with CLIA, CLSI, CAP, COLA, and applicable state regulations, and for determining the appropriateness of study design and acceptance criteria for their specific test systems.
Designed for small laboratories and physician office laboratories (POLs), this Excel-based template supports user verification of precision for FDA-cleared, non-waived quantitative tests in accordance with CLSI EP15.
Precision verification is a required component of method verification, yet many smaller labs perform these studies infrequently and do not need the cost or complexity of enterprise statistical software. This template provides a practical, cost-effective alternative, offering the structure, calculations, and documentation support needed to perform CLSI-aligned precision verification without ongoing software subscriptions.
The study design incorporates multiple runs and multiple days, capturing both within-run and between-run sources of variability through its structure and allowing laboratories to evaluate overall within-laboratory precision, consistent with CLSI EP15 expectations. Separate variance component analysis is not required for EP15 verification.
The template includes clearly organized data entry fields, automated calculations, and objective acceptance logic to support precision verification during new test implementation, instrument changes, or other verification activities.
Key Features
Structured worksheet supporting CLSI EP15 precision verification
Multi-day, multi-run design that incorporates within-run and between-run variability
Automated calculations for:
Mean
Standard deviation (SD)
Coefficient of variation (CV%)
Automatic PASS / FAIL determination based on manufacturer precision claims
Clearly defined study design section for inspection clarity
Separate Instructions tab explaining study design, data entry, and interpretation
Clean, professional format suitable for CLIA, CAP, TJC, and COLA inspections
Designed by a CLSI-experienced Clinical Laboratory Scientist and Laboratory Director
Reusable for multiple analytes and instruments
Why This Template Makes Sense for Small Labs and POLs
Many small laboratories and physician office labs do not need to maintain annual licenses for statistical software just to perform occasional precision verification studies. This template is designed for laboratories that need reliable, CLSI-aligned documentation without unnecessary overhead.
For labs that:
Perform verification studies periodically rather than continuously
Operate with limited testing volume
Value practical, inspection-ready documentation
this template offers the right level of rigor without unnecessary cost or complexity.
Intended Use
This spreadsheet is intended to assist clinical laboratories with internal documentation and evaluation of precision verification studies for moderate- and high-complexity testing, when verification is appropriate for FDA-cleared test systems and within the laboratory’s scope of practice.
This template supports precision verification (CLSI EP15) and is not intended to replace full precision characterization studies described in CLSI EP05.
Compatibility
Microsoft Excel (desktop version recommended)
Not designed for Google Sheets or Excel Online
License
Single-laboratory use
Redistribution, resale, or sharing outside the licensed laboratory is prohibited
Disclaimer
This template is provided as an educational and documentation support tool and does not replace professional judgment or regulatory requirements. Laboratories are responsible for ensuring compliance with CLIA, CLSI, CAP, COLA, and applicable state regulations, and for determining the appropriateness of study design and acceptance criteria for their specific test systems.
